Non-Metastatic Esophageal Adenocarcinoma: Circulating Tumor Cells in the Course of Multimodal Tumor Treatment
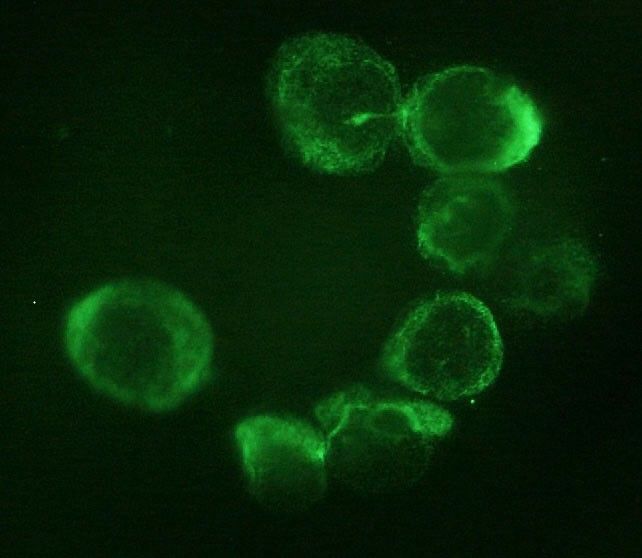
Background: Isolation of circulating tumor cells (CTC) holds the promise to improve response-prediction and personalization of cancer treatment. In this study, we test a filtration device for CTC isolation in patients with non-metastatic esophageal adenocarcinoma (EAC) within recent multimodal treatment protocols.
Methods: Peripheral blood specimens were drawn from EAC patients before and after neoadjuvant chemotherapy (FLOT)/chemoradiation (CROSS) as well as after surgery. Filtration using ScreenCell® devices captured CTC for cytologic analysis. Giemsa-stained specimens were evaluated by a cytopathologist; the cut-off was 1 CTC/specimen (6 mL). Immunohistochemistry with epithelial (pan-CK) and mesenchymal markers (vimentin) was performed.
Results: Morphologically diverse malignant CTCs were found in 12/20 patients in at least one blood specimen. CTCs were positive for both vimentin and pan-CK. More patients were CTC positive after neoadjuvant therapy (6/20 vs. 9/15) and CTCs per/ml increased in most of the CTC-positive patients. After surgery, 8/13 patients with available blood specimens were still CTC positive. In clinical follow-up, 5/9 patients who died were CTC-positive.
Conclusions: Detection of CTC by filtration within multimodal treatment protocols of non-metastatic EAC is feasible. The rate of CTC positive findings and the quantity of CTCs changes in the course of multimodal neoadjuvant chemoradiation/chemotherapy and surgery.
Cytopathological Heterogeneity of Circulating Tumor Cells in Non-metastatic Esophageal Adenocarcinoma

Background/aim: The presence of circulating tumor cells (CTC) has been reported to have an impact on prognosis in different tumor entities. Little is known about CTC morphology and heterogeneity.
Patients and methods: In a multicenter setting, pre-therapeutic peripheral blood specimens were drawn from patients with non-metastatic esophageal adenocarcinoma (EAC). CTCs were captured by size-based filtration (ScreenCell®), subsequently Giemsa-stained and evaluated by two trained readers. The isolated cells were categorized in groups based on morphologic criteria.
Results: Small and large single CTCs, as well as CTC-clusters, were observed in 69.2% (n=81) of the 117 specimens; small CTCs were observed most frequently (59%; n=69), followed by large CTCs (40%; n=47) and circulating cancer-associated macrophage-like cells (CAMLs; 34.2%, n=40). Clusters were rather rare (12%; n=14). CTC/CAML were heterogeneous in the cohort, but also within one specimen. Neither the presence of the CTC subtypes/CAMLs nor the exact cell count were associated with the primary clinical TNM stage.
Conclusion: Morphologically heterogenic CTCs and CAMLs are present in patients with non-metastatic, non-pretreated EAC.
A Fast and Furious Liquid Biopsy Assay to Monitor Targeted Therapy Resistance
Liquid biopsies represent a valid alternative to conventional tissue biopsies, offering a real time molecular picture of tumors in a minimally invasive manner. Of the various circulating biomarkers available for liquid biopsy, circulating tumor cells (CTC) and circulating tumor DNA (ctDNA) are the most intensively studied to date. However, CTC and ctDNA represent different tumor components, therefore, complementary information from both sources might be beneficial. This protocol focuses on the description of a sample processing workflow that allowed for concurrent isolation of CTC and ctDNA from the same source sample. This single tube approach enables simultaneous analysis of multiple biomarkers to better monitor cancer drug resistance.
Circulating Tumor Cells in Diagnosing Lung Cancer: Clinical and Morphologic Analysis
Background
The purpose of this study was to evaluate the value of circulating non-hematologic cells to differentiate benign from malignant lung lesions and their comparison with clinico-histologic features of corresponding primary lesions.
Methods
Circulating cells were isolated by size method from peripheral blood of 77 patients with malignant (n = 60) and benign (n = 17) lung lesions. They were morphologically classified as cells with malignant feature; cells with uncertain malignant feature; and cells with benign feature; then statistically correlated with clinico-cytopathologic characteristics of corresponding lung lesion.
Results
Malignant circulating cells were detected in 54 of 60 (90%) malignant patients, and in 1 of 17 (5%) benign patients; benign circulating cells in 1 of 60 (1%) malignant patients and in 15 of 17 (88%) benign patients; and circulating cells with uncertain malignant aspect in 5 of 60 (8%) malignant patients and 1 of 17 (5%) benign patients. For a malignant circulating cells count greater than 25, sensitivity and specificity were 89% and 100%, respectively. The count was significantly correlated with stage, size, and standard uptake value of primary tumor. In 39 of 54 (72%) cases, the malignant circulating cells allowed a specific histologic diagnosis of the corresponding primary tumor after immunohistochemical analysis.
Conclusions
Malignant circulating cells may be a valid marker in the diagnostic workup of lung lesions. However, our resuts should be corroborated by larger future studies especially for patients having small nodules.
An assessment of diagnostic performance of a filter-based antibody-independent peripheral blood circulating tumour cell capture paired with cytomorphologic criteria for the diagnosis of cancer. Lung Cancer

Objectives
Circulating tumour cells (CTCs) are reported to be predictive for prognosis and response to treatment in advanced lung cancer. However, the clinical utility of the CTCs detection remains unknown for early stage lung cancer as the number of CTCs is reported as low, providing challenges in identification. We have evaluated diagnostic performance of filtration-based technology using cytomorphologic criteria in patients undergoing surgery for lung cancer.
Material and methods
We processed blood from 76 patients undergoing surgery for known or suspected lung cancer using ScreenCell® Cyto filter devices. Captured cells were stained using haematoxylin and eosin and independently assessed by two pathologists for the presence of atypical cells suspicious for cancer. Diagnostic performance was evaluated against pathologist reported diagnoses of cancer from surgically obtained specimens.
Results
Cancer was diagnosed in 57 patients (77.0%), including 32 with primary lung cancer (56.1%). The proportion of patients with early stage primary lung cancer in which CTCs were identified was 18 and 21 (56.3% and 65.6%, respectively) as reported by two pathologists. The agreement between the pathologists was 77.0% corresponding to a kappa-statistic of 53.7% indicating moderate agreement. No significant differences were found for the percentage of CTCs for primary and metastatic cancer as well as for cancer stages. On sensitivity weighted analysis, a sensitivity and specificity were 71.9% (95% CI 60.5–83.0) and 52.9% (95% CI 31.1–77.0), respectively. On specificity weighted analysis, a sensitivity and specificity were 50.9% (95% CI 39.3–64.4) and 82.4% (60.4–96.2), respectively.
Conclusion
The performance of the tested filter-based antibody-independent technology to capture CTCs using standard cytomorphologic criteria provides the potential of a diagnostic blood test for lung cancer.
Usefulness of circulating tumor cell detection in pancreatic adenocarcinoma diagnosis
No abstract available
Circulating tumor cells found in patients with localized and advanced pancreatic cancer

Objectives
Isolation of circulating tumor cells (CTCs) holds the promise of diagnosing and molecular profiling cancers from a blood sample. Here, we test a simple new low-cost filtration device for CTC isolation in patients with pancreatic ductal adenocarcinoma (PDAC).
Methods
Peripheral blood samples drawn from healthy donors and PDAC patients were filtered using ScreenCell devices, designed to capture CTCs for cytologic and molecular analysis. Giemsa-stained specimens were evaluated by a pancreatic cytopathologist blinded to the histological diagnosis. Circulating tumor cell DNA was subjected to KRAS mutational analysis.
Results
Spiking experiments demonstrated a CTC capture efficiency as low as 2 cells/mL of blood. Circulating tumor cells were identified by either malignant cytology or presence of KRAS mutation in 73% of 11 patients (P = 0.001). Circulating tumor cells were identified in 3 of 4 patients with early (≤American Joint Committee on Cancer stage IIB) and in 5 of 7 patients with advanced (≥ American Joint Committee on Cancer stage III) PDAC. No CTCs were detected in blood from 9 health donors.
Conclusions
Circulating tumor cells can be found in most patients with PDAC of any stage, whether localized, locally advanced, or metastatic. The ability to capture, cytologically identify, and genetically analyze CTCs suggests a possible tool for the diagnosis and characterization of genetic alterations of PDAC.
Cluster circulating tumor cells in surgical cases of lung cancer

Objectives: A cancer lesion sheds tumor cells into the circulating blood as circulating tumor cells (CTCs). Since cluster CTCs have been considered as precursor lesions of metastasis, their clinical implication was investigated in this study according to the preoperative status of cluster CTC detection in surgical cases of clinically early-stage lung cancer.
Methods: Among 104 surgical patients of early-stage lung cancer, CTCs were extracted from the peripheral blood before surgery using a micro-pore size selection method (ScreenCell®) and diagnosed microscopically. Implications of detecting cluster CTC were assessed according to the prognosis and clinicopathological characteristics.
Results: The status of CTC detection was not detected in 77 cases (74.0%), single CTC only detection in 7 cases (6.7%), and cluster CTC detected in 20 cases (19.2%). Patients with cluster CTCs exhibited significantly lower recurrence-free survival and overall survival than did patients of other groups. In addition, in hazard ratio analysis, the hazard ratios were independent of other predictors of poor prognosis, and detection of cluster CTCs was associated with predictors of poor prognosis.
Conclusion: Cluster CTCs were detected in cases where the original lung cancer lesion had clinical predictors of poor prognosis and were independent negative predictors of survival.
The Prognostic Value of the Circulating Tumor Cell-Based Four mRNA Scoring System: A New Non-Invasive Setting for the Management of Bladder Cancer
Bladder cancer (BC) is one of the most expensive lifetime cancers to treat because of the high recurrence rate, repeated surgeries, and long-term cystoscopy monitoring and treatment. The lack of an accurate classification system predicting the risk of recurrence or progression leads to the search for new biomarkers and strategies. Our pilot study aimed to identify a prognostic gene signature in circulating tumor cells (CTCs) isolated by ScreenCell devices from muscle invasive and non-muscle invasive BC patients. Through the PubMed database and Cancer Genome Atlas dataset, a panel of 15 genes modulated in BC with respect to normal tissues was selected. Their expression was evaluated in CTCs and thanks to the univariate and multivariate Cox regression analysis, EGFR, TRPM4, TWIST1, and ZEB1 were recognized as prognostic biomarkers. Thereafter, by using the risk score model, we demonstrated that this 4-gene signature significantly grouped patients into high- and low-risk in terms of recurrence free survival (HR = 2.704, 95% CI = 1.010−7.313, Log-rank p < 0.050). Overall, we identified a new prognostic signature that directly impacted the prediction of recurrence, improving the choice of the best treatment for BC patients.
Gene signatures of circulating breast cancer cell models are a source of novel molecular determinants of metastasis and improve circulating tumor cell detection in patients
Background: Progression to stage IV disease remains the main cause of breast cancer-related deaths. Increasing knowledge on the hematogenous phase of metastasis is key for exploiting the entire window of opportunity to interfere with early dissemination and to achieve a more effective disease control. Recent evidence suggests that circulating tumor cells (CTCs) possess diverse adaptive mechanisms to survive in blood and eventually metastasize, encouraging research into CTC-directed therapies.
Methods: On the hypothesis that the distinguishing molecular features of CTCs reveal useful information on metastasis biology and disease outcome, we compared the transcriptome of CTCs, primary tumors, lymph-node and lung metastases of the MDA-MB-231 xenograft model, and assessed the biological role of a panel of selected genes, by in vitro and in vivo functional assays, and their clinical significance in M0 and M+ breast cancer patients.
Results: We found that hematogenous dissemination is governed by a transcriptional program and identified a CTC signature that includes 192 up-regulated genes, mainly related to cell plasticity and adaptation, and 282 down-regulated genes, involved in chromatin remodeling and transcription. Among genes up-regulated in CTCs, FADS3 was found to increases cell membrane fluidity and promote hematogenous diffusion and lung metastasis formation. TFF3 was observed to be associated with a subset of CTCs with epithelial-like features in the experimental model and in a cohort of 44 breast cancer patients, and to play a role in cell migration, invasion and blood-borne dissemination. The analysis of clinical samples with a panel of CTC-specific genes (ADPRHL1, ELF3, FCF1, TFF1 and TFF3) considerably improved CTC detection as compared with epithelial and tumor-associated markers both in M0 and stage IV patients, and CTC kinetics informed disease relapse in the neoadjuvant setting.
Conclusions: Our findings provide evidence on the potential of a CTC-specific molecular profile as source of metastasis-relevant genes in breast cancer experimental models and in patients. Thanks to transcriptome analysis we generated a novel CTC signature in the MDA-MB-231 xenograft model, adding a new piece to the current knowledge on the key players that orchestrate tumor cell hematogenous dissemination and breast cancer metastasis, and expanding the list of CTC-related biomarkers for future validation studies.