Detection of circulating tumor cells in patients with adrenocortical carcinoma: a monocentric preliminary study
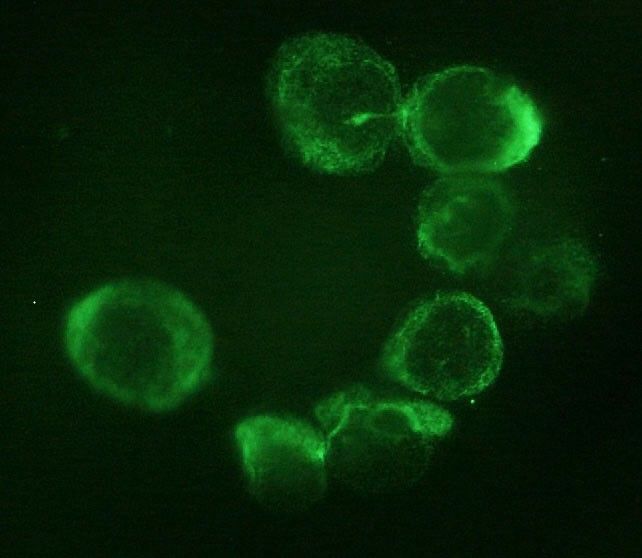
Context: Adrenocortical carcinoma (ACC) is a rare malignancy, the prognosis of which is mainly dependent on stage at diagnosis. The identification of disease-associated markers for early diagnosis and drug monitoring is mandatory. Circulating tumor cells (CTCs) are released into the bloodstream from primary tumor/metastasis. CTC detection in blood samples may have enormous potential for assisting in the diagnosis of malignancy, estimating prognosis, and monitoring the disease.
Objective: The aim of the study was to investigate the presence of CTCs in blood samples of patients with ACC or benign adrenocortical adenoma (ACA).
Setting: We conducted the study at a university hospital.
Intervention: CTC analysis was performed in blood samples from 14 ACC patients and 10 ACA patients. CTCs were isolated on the basis of cell size by filtration through ScreenCell devices, followed by identification according to validated morphometric criteria and immunocytochemistry.
Main outcome measure: We measured the difference in CTC detection between ACC and ACA.
Results: CTCs were detected in all ACC samples, but not in ACA samples. Immunocytochemistry confirmed the adrenocortical origin. When ACC patients were stratified according to the median value of tumor diameter and metastatic condition, a statistically significant difference was found in the number of CTCs detected after surgery. A significant correlation between the number of CTCs in postsurgical samples and clinical parameters was found for tumor diameter alone.
Conclusions: Our findings provide the first evidence for adrenocortical tumors that CTCs may represent a useful marker to support differential diagnosis between ACC and ACA. The correlation with some clinical parameters suggests a possible relevance of CTC analysis for prognosis and noninvasive monitoring of disease progression and drug response.
Thoracic surgery in the UK

No abstract available
A new device for rapid isolation by size and characterization of rare circulating tumor cells

Background: Circulating tumor cells (CTCs) likely derive from clones in the primary tumor, suggesting that they can be used for all biological tests applying to the primary cells. Materials and Methods: The ScreenCell® devices are single-use and low-cost innovative devices that use a filter to isolate and sort tumor cells by size. Results: The ScreenCell® Cyto device is able to isolate rare, fixed, tumor cells, with a high recovery rate. Cells are well preserved morphologically. Immunocytochemistry and FISH assays can be performed directly on the filter. The ScreenCell® CC device allows isolation of live cells able to grow in culture. High quality genetic materials can be obtained directly from tumor cells isolated on the ScreenCell® MB device filter. Conclusion: Due to their reduced size, versatility, and capacity to isolate CTCs within minutes, the ScreenCell® devices may be able to simplify and improve non-invasive access to tumor cells.
Rapid and Simple Isolation of Circulating Tumor Cells for Clinical and Research Applications Using ScreenCell

Circulating tumor cells (CTCs) are malignant cells shed by the primary tumor or metastases into the peripheral circulation. CTCs give rise to distant metastases that are usually the ultimate cause of cancer-related death. A significant proportion of patients with early stage cancer in whom no metastases are identifiable will ultimately relapse as a result of hematogenous spread of tumor cells that were undetected at initial diagnosis and treatment.
Rapid separation of mononuclear hodgkin from multinuclear reed-sternberg cells

We describe a method to isolate small mononucleated Hodgkin (H) cells from multinucleated Reed Sternberg (RS) cells of Hodgkin lymphoma using the ScreenCell filter device. This filtration-based approach lends itself to future clinical applications in that it enables the separation of H and RS cells from lymph node biopsies, bone marrow aspirates, pleural effusions, and blood, including the isolation of monoclonal Hodgkin precursor cells from the blood.
Non-Metastatic Esophageal Adenocarcinoma:Circulating Tumor Cells in the Course of Multimodal Tumor Treatment

Background: Isolation of circulating tumor cells (CTC) holds the promise to improve response-prediction and personalization of cancer treatment. In this study, we test a filtration device for CTC isolation in patients with non-metastatic esophageal adenocarcinoma (EAC) within recent multimodal treatment protocols.
Methods: Peripheral blood specimens were drawn from EAC patients before and after neoadjuvant chemotherapy (FLOT)/chemoradiation (CROSS) as well as after surgery. Filtration using ScreenCell® devices captured CTC for cytologic analysis. Giemsa-stained specimens were evaluated by a cytopathologist; the cut-off was 1 CTC/specimen (6 mL). Immunohistochemistry with epithelial (pan-CK) and mesenchymal markers (vimentin) was performed.
Results: Morphologically diverse malignant CTCs were found in 12/20 patients in at least one blood specimen. CTCs were positive for both vimentin and pan-CK. More patients were CTC positive after neoadjuvant therapy (6/20 vs. 9/15) and CTCs per/ml increased in most of the CTC-positive patients. After surgery, 8/13 patients with available blood specimens were still CTC positive. In clinical follow-up, 5/9 patients who died were CTC-positive.
Conclusions: Detection of CTC by filtration within multimodal treatment protocols of non-metastatic EAC is feasible. The rate of CTC positive findings and the quantity of CTCs changes in the course of multimodal neoadjuvant chemoradiation/chemotherapy and surgery.
Usefulness of circulating tumor cell detection in pancreatic adenocarcinoma diagnosis.
No abstract available
Strategies for Isolating and Propagating Circulating Tumor Cells in Men with Metastatic Prostate Cancer
Selecting a well-suited method for isolating/characterizing circulating tumor cells (CTCs) is challenging. Evaluating sensitive and specific markers for prostate cancer (PCa)-specific CTC identification and analysis is crucial. We used the CellCollector EpCAM-functionalized system (CC-EpCAM) and evaluated and developed a PCa-functionalized version (CC-PCa); we then compared CTC isolation techniques that exploit the physical and biological properties of CTCs. We established two cohorts of metastatic PCa patients (mPCa; 15 in cohort 1 and 10 in cohort 2). CTC cultivation experiments were conducted with two capturing methods (Ficoll and ScreenCell). The most sensitive detection rates and highest CTC counts were reached with the CC-PCa and ScreenCell system. Patients with ≥5 CTCs isolated with CC-EpCAM had an overall survival (OS) of 0.93 years, and patients with ≥5 CTCs isolated with CC-PCa had an OS of 1.5 years in cohort 1. Nevertheless, we observed the highest sensitivity and specificity for 24-month survival by the Ficoll with CD45 depletion and ScreenCell system with May-Grunwald Giemsa (MGG) staining. The EpCAM molecule is an essential factor related to OS for CTC isolation based on biological properties in mPCa patients. The best-suited CTC capture system is not limited to one characteristic of cells but adapted to downstream analysis.
A Fast and Furious Liquid Biopsy Assay to Monitor Targeted Therapy Resistance.
Liquid biopsies represent a valid alternative to conventional tissue biopsies, offering a real time molecular picture of tumors in a minimally invasive manner. Of the various circulating biomarkers available for liquid biopsy, circulating tumor cells (CTC) and circulating tumor DNA (ctDNA) are the most intensively studied to date. However, CTC and ctDNA represent different tumor components, therefore, complementary information from both sources might be beneficial. This protocol focuses on the description of a sample processing workflow that allowed for concurrent isolation of CTC and ctDNA from the same source sample. This single tube approach enables simultaneous analysis of multiple biomarkers to better monitor cancer drug resistance.
The Prognostic Value of the Circulating Tumor Cell-Based Four mRNA Scoring System: A New Non-Invasive Setting for the Management of Bladder Cancer
Bladder cancer (BC) is one of the most expensive lifetime cancers to treat because of the high recurrence rate, repeated surgeries, and long-term cystoscopy monitoring and treatment. The lack of an accurate classification system predicting the risk of recurrence or progression leads to the search for new biomarkers and strategies. Our pilot study aimed to identify a prognostic gene signature in circulating tumor cells (CTCs) isolated by ScreenCell devices from muscle invasive and non-muscle invasive BC patients. Through the PubMed database and Cancer Genome Atlas dataset, a panel of 15 genes modulated in BC with respect to normal tissues was selected. Their expression was evaluated in CTCs and thanks to the univariate and multivariate Cox regression analysis, EGFR, TRPM4, TWIST1, and ZEB1 were recognized as prognostic biomarkers. Thereafter, by using the risk score model, we demonstrated that this 4-gene signature significantly grouped patients into high- and low-risk in terms of recurrence free survival (HR = 2.704, 95% CI = 1.010−7.313, Log-rank p < 0.050). Overall, we identified a new prognostic signature that directly impacted the prediction of recurrence, improving the choice of the best treatment for BC patients.
