A Fast and Furious Liquid Biopsy Assay to Monitor Targeted Therapy Resistance.
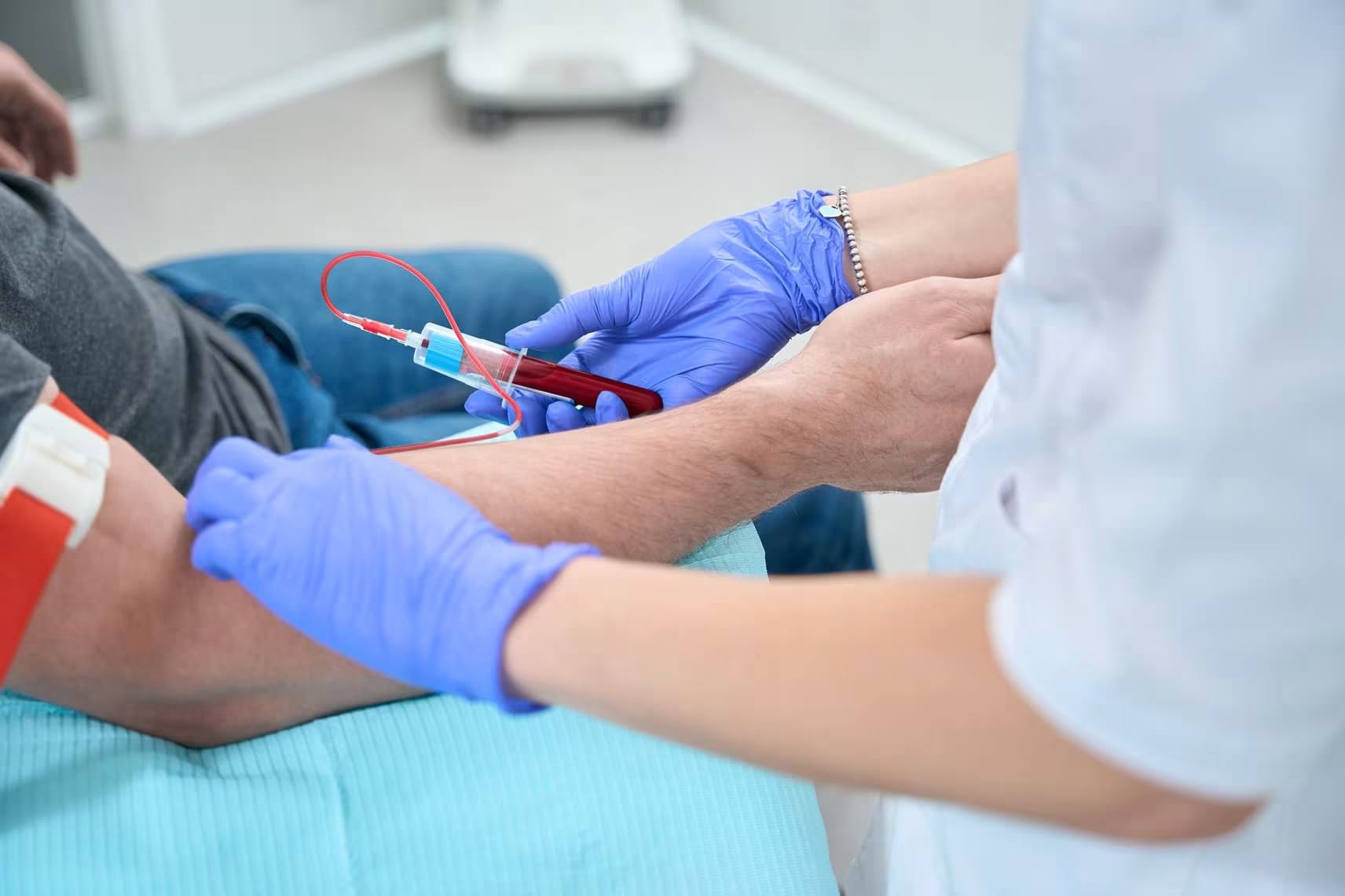
Liquid biopsies represent a valid alternative to conventional tissue biopsies, offering a real time molecular picture of tumors in a minimally invasive manner. Of the various circulating biomarkers available for liquid biopsy, circulating tumor cells (CTC) and circulating tumor DNA (ctDNA) are the most intensively studied to date. However, CTC and ctDNA represent different tumor components, therefore, complementary information from both sources might be beneficial. This protocol focuses on the description of a sample processing workflow that allowed for concurrent isolation of CTC and ctDNA from the same source sample. This single tube approach enables simultaneous analysis of multiple biomarkers to better monitor cancer drug resistance.
The Prognostic Value of the Circulating Tumor Cell-Based Four mRNA Scoring System: A New Non-Invasive Setting for the Management of Bladder Cancer

Simple Summary: Bladder cancer with similar diagnosis based on traditional classification exhibits different behaviors and therapeutic outcomes. Thus, circulating tumor cells (CTCs) represent a more accurate approach to investigate bladder cancer features. Our results demonstrate that risk score based on EGFR, TRPM4, TWIST1, and ZEB1 four-gene signature in CTCs is markedly and undoubtedly associated with recurrence, suggesting an innovative and non-invasive strategy to manage both non muscle invasive and muscle invasive bladder cancer progression without the
necessity of repetitive and onerous cystoscopies.
Abstract: Bladder cancer (BC) is one of the most expensive lifetime cancers to treat because of the high recurrence rate, repeated surgeries, and long-term cystoscopy monitoring and treatment. The lack of an accurate classification system predicting the risk of recurrence or progression leads to the search for new biomarkers and strategies. Our pilot study aimed to identify a prognostic gene signature in circulating tumor cells (CTCs) isolated by ScreenCell devices from muscle invasive and non-muscle invasive BC patients. Through the PubMed database and Cancer Genome Atlas dataset, a panel
of 15 genes modulated in BC with respect to normal tissues was selected. Their expression was evaluated in CTCs and thanks to the univariate and multivariate Cox regression analysis, EGFR, TRPM4, TWIST1, and ZEB1 were recognized as prognostic biomarkers. Thereafter, by using the risk score model, we demonstrated that this 4-gene signature significantly grouped patients into high- and low-risk in terms of recurrence free survival (HR = 2.704, 95% CI = 1.010–7.313, Log-rank p < 0.050). Overall, we identified a new prognostic signature that directly impacted the prediction of recurrence, improving the choice of the best treatment for BC patients.
Strategies for Isolating and Propagating Circulating Tumor Cells in Men with Metastatic Prostate Cancer
Selecting a well-suited method for isolating/characterizing circulating tumor cells (CTCs) is challenging. Evaluating sensitive and specific markers for prostate cancer (PCa)-specific CTC identification and analysis is crucial. We used the CellCollector EpCAM-functionalized system (CC-EpCAM) and evaluated and developed a PCa-functionalized version (CC-PCa); we then compared CTC isolation techniques that exploit the physical and biological properties of CTCs. We established two cohorts of metastatic PCa patients (mPCa; 15 in cohort 1 and 10 in cohort 2). CTC cultivation experiments were conducted with two capturing methods (Ficoll and ScreenCell). The most sensitive detection rates and highest CTC counts were reached with the CC-PCa and ScreenCell system. Patients with ≥5 CTCs isolated with CC-EpCAM had an overall survival (OS) of 0.93 years, and patients with ≥5 CTCs isolated with CC-PCa had an OS of 1.5 years in cohort 1. Nevertheless, we observed the highest sensitivity and specificity for 24-month survival by the Ficoll with CD45 depletion and ScreenCell system with May-Grunwald Giemsa (MGG) staining. The EpCAM molecule is an essential factor related to OS for CTC isolation based on biological properties in mPCa patients. The best-suited CTC capture system is not limited to one characteristic of cells but adapted to downstream analysis.
Détection des mutations RAS dans les cellules tumorales circulantes : applications au cancer colorectal et perspectives

Les mutations somatiques présentes dans les gènes RAS (KRAS et NRAS) sont largement associées à l’absence de réponse aux traitements par immunothérapie ciblant le récepteur du facteur de croissance épidermique dans le cancer colorectal métastatique. La recherche de ces mutations est devenue obligatoire pour pouvoir prescrire ces traitements et cette détection est réalisée à partir de biopsies tissulaires. Dans environ 25 % des cas, cette analyse n’est pas possible pour des raisons à la fois analytique et médicale et par conséquent le développement de stratégies alternatives est donc nécessaire. Parmi les solutions envisagées, la recherche de mutations directement dans le sang des patients est une approche prometteuse. Parmi les sources d’ADN tumoral présent dans la circulation sanguine, les cellules tumorales circulantes (CTC) représentent une approche particulièrement pertinente. Ces cellules, dont certaines sont à l’origine des métastases, sont parvenues à se détacher de la tumeur primitive, puis à migrer et enfin à entrer dans le système vasculaire. En ce sens, elles sont particulièrement résistantes aux contraintes physico-chimiques et immunologiques mises en œuvre par l’organisme pour empêcher leur dissémination et représentent par conséquent une source d’informations particulièrement précieuse sur la génétique des cellules tumorales les plus agressives. Le corollaire est que ces cellules sont très rares et nécessitent des technologies particulièrement performantes pour les détecter et les caractériser. Dans cette présentation, nous nous focaliserons principalement sur les méthodes moléculaires permettant de détecter les mutations des gènes RAS sur les CTC en analysant les performances d’une solution basée sur une méthode d’enrichissement par filtration suivi d’une détection par PCR digitale. Enfin, nous nous interrogerons sur leur signification biologique avant d’évoquer leurs perspectives dans le cancer colorectal ainsi que dans d’autres types de cancers.
Rapid and Sensitive Detection of Breast Cancer Cells in Patient Blood with Nuclease-Activated Probe Technology

A challenge for circulating tumor cell (CTC)-based diagnostics is the development of simple and inexpensive methods that reliably detect the diverse cells that make up CTCs. CTC-derived nucleases are one category of proteins that could be exploited to meet this challenge. Advantages of nucleases as CTC biomarkers include: (1) their elevated expression in many cancer cells, including cells implicated in metastasis that have undergone epithelial-to-mesenchymal transition; and (2) their enzymatic activity, which can be exploited for signal amplification in detection methods. Here, we describe a diagnostic assay based on quenched fluorescent nucleic acid probes that detect breast cancer CTCs via their nuclease activity. This assay exhibited robust performance in distinguishing breast cancer patients from healthy controls, and it is rapid, inexpensive, and easy to implement in most clinical labs. Given its broad applicability, this technology has the potential to have a substantive impact on the diagnosis and treatment of many cancers.
Droplet digital PCR of circulating tumor cells from colorectal cancer patients can predict KRAS mutations before surgery

In colorectal cancer (CRC), KRAS mutations are a strong negative predictor for treatment with the EGFR-targeted antibodies cetuximab and panitumumab. Since it can be difficult to obtain appropriate tumor tissues for KRAS genotyping, alternative methods are required. Circulating tumor cells (CTCs) are believed to be representative of the tumor in real time. In this study we explored the capacity of a size-based device for capturing CTCs coupled with a multiplex KRAS screening assay using droplet digital PCR (ddPCR). We showed that it is possible to detect a mutant ratio of 0.05% and less than one KRAS mutant cell per mL total blood with ddPCR compared to about 0.5% and 50–75 cells for TaqMeltPCR and HRM. Next, CTCs were isolated from the blood of 35 patients with CRC at various stage of the disease. KRAS genotyping was successful for 86% (30/35) of samples with a KRAS codon 12/13 mutant ratio of 57% (17/30). In contrast, only one patient was identified as KRAS mutant when size-based isolation was combined with HRM or TaqMeltPCR. KRAS status was then determined for the 26 available formalin-fixed paraffin-embedded tumors using standard procedures. The concordance between the CTCs and the corresponding tumor tissues was 77% with a sensitivity of 83%. Taken together, the data presented here suggest that is feasible to detect KRAS mutations in CTCs from blood samples of CRC patients which are predictive for those found in the tumor. The minimal invasive nature of this procedure in combination with the high sensitivity of ddPCR might provide in the future an opportunity to monitor patients throughout the course of disease on multiple levels including early detection, prognosis, treatment and relapse as well as to obtain mechanistic insight with respect to tumor invasion and metastasis.
A new device for rapid isolation by size and characterization of rare circulating tumor cells

Background: Circulating tumor cells (CTCs) likely derive from clones in the primary tumor, suggesting that they can be used for all biological tests applying to the primary cells.
Materials and methods: The ScreenCell® devices are single-use and low-cost innovative devices that use a filter to isolate and sort tumor cells by size.
Results: The ScreenCell® Cyto device is able to isolate rare, fixed, tumor cells, with a high recovery rate. Cells are well preserved morphologically. Immunocytochemistry and FISH assays can be performed directly on the filter. The ScreenCell® CC device allows isolation of live cells able to grow in culture. High quality genetic materials can be obtained directly from tumor cells isolated on the ScreenCell® MB device filter.
Conclusion: Due to their reduced size, versatility, and capacity to isolate CTCs within minutes, the ScreenCell® devices may be able to simplify and improve non-invasive access to tumor cells.